VSEPR
Jump to navigation
Jump to search
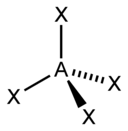
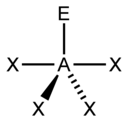
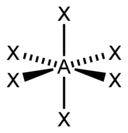
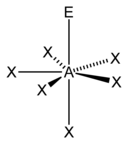
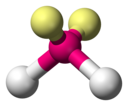
VSEPR Geometries
[edit]Valence shell electron pair repulsion (VSEPR) theory (1957) is a model in chemistry, which is used for predicting the shapes of individual molecules, based upon their extent of electron-pair electrostatic repulsion, determined using steric numbers.
2D
[edit]