File:Amine to Ammonium.PNG
From Wikimedia Commons, the free media repository
Jump to navigation
Jump to search
Amine_to_Ammonium.PNG (350 × 165 pixels, file size: 3 KB, MIME type: image/png)
File information
Structured data
Captions
Captions
Add a one-line explanation of what this file represents
| DescriptionAmine to Ammonium.PNG | Chemical Reaction: Amine neutralized by acid (H+) to form Substituted-Ammonium Cation |
| Date | |
| Source | H Padleckas created this image file (finished on January 22, 2005) especially for the article "Amine" in Wikimedia. H Padleckas 19:58, 26 Jan 2005 (UTC) |
| Author | H Padleckas |
|
This chemical image could be re-created using vector graphics as an SVG file. This has several advantages; see Commons:Media for cleanup for more information. If an SVG form of this image is available, please upload it and afterwards replace this template with
{{vector version available|new image name}}.
It is recommended to name the SVG file “Amine to Ammonium.svg”—then the template Vector version available (or Vva) does not need the new image name parameter. |
| Public domainPublic domainfalsefalse |
| I, the copyright holder of this work, release this work into the public domain. This applies worldwide. In some countries this may not be legally possible; if so: I grant anyone the right to use this work for any purpose, without any conditions, unless such conditions are required by law. |
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | Thumbnail | Dimensions | User | Comment | |
|---|---|---|---|---|---|
| current | 04:06, 14 June 2009 |  | 350 × 165 (3 KB) | H Padleckas (talk | contribs) | changed R_1, R_2, R_3 to R, R', R'' |
| 18:30, 28 January 2005 | 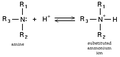 | 350 × 165 (3 KB) | H Padleckas (talk | contribs) | made it a reversible reaction | |
| 19:45, 26 January 2005 |  | 350 × 165 (3 KB) | H Padleckas (talk | contribs) | Amine neutralized by acid to Substituted-Ammonium Cation |
You cannot overwrite this file.
File usage on Commons
There are no pages that use this file.
File usage on other wikis
The following other wikis use this file:
- Usage on pt.wikipedia.org